Regulatory Process For Medical Devices In Europe
Union Register of medicinal products - Public health - European Commission. European Commission. Live, work, travel in the EU. Union Register support.
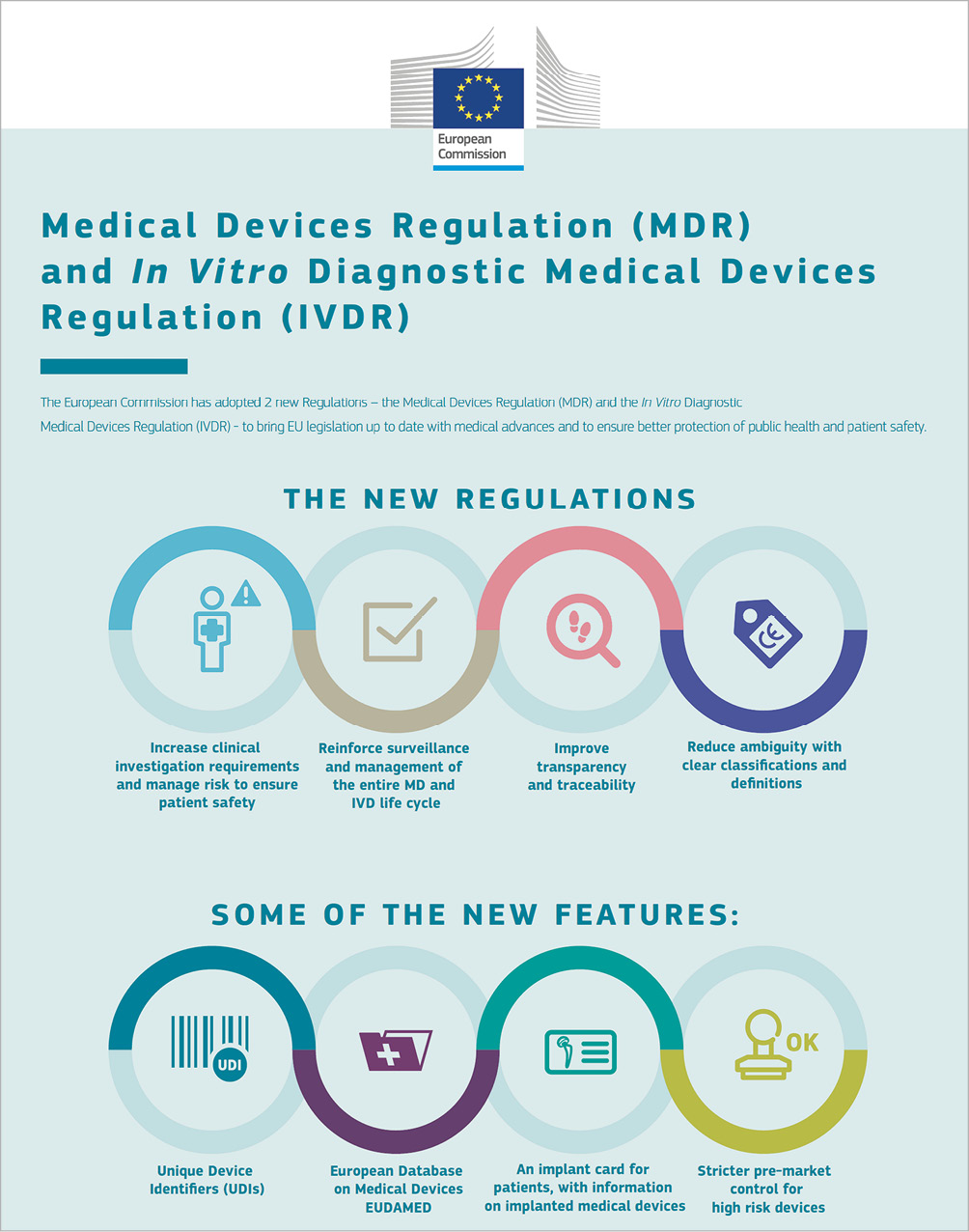
Medical device regulation in Europe what is changing and how can I more involved
The European Union has targeted another Chinese company under its foreign subsidies regulation, the Post can reveal. On Tuesday morning, EU officials entered the premises of the Dutch and Polish.
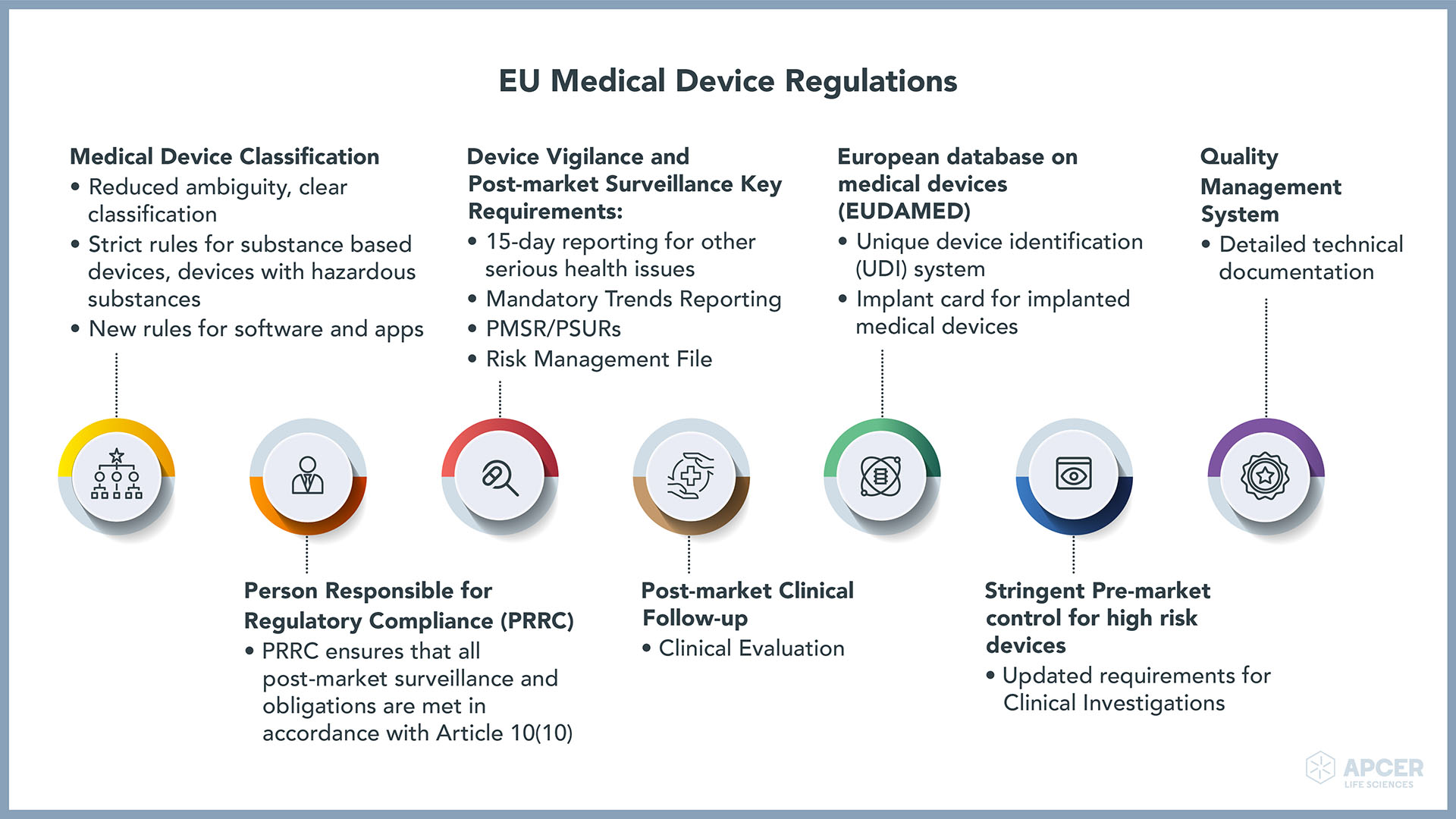
EU Medical Device Regulations APCER Life Sciences
The following new legislation is applicable within the EU. 26 May 2021: Regulation (EU) 2017/745 on medical devices 26 May 2022: Regulation (EU) 2017/746 on in vitro diagnostic medical devices The EU has revised the legal framework of the current 3 directives to reflect progress over the last 20 years. Adopted in May 2017, the new rules will fully apply after the transitional periods provided.

The New EU’s Medical Devices Regulation (MDR) entered into force last month, it set in motion a
Publication of Regulation (EU) 2023/607 amending Regulations (EU) 2017/745 and (EU) 2017/746 as regards the transitional provisions for certain medical devices and in vitro diagnostic medical devices. Publication of a Q&A on practical aspects related to the implementation of Regulation (EU) 2023/607. February 2023:

European Union Medical Device Directive (MDD) to Medical Device Regulations (MDR) strategic
The Regulations on Medical Devices (Regulation (EU) 2017/745) and on In Vitro Diagnostic Devices (Regulation (EU) 2017/746) changed the European legal framework for medical devices, introducing new responsibilities for EMA and national competent authorities in the assessment of certain categories of medical device.. The Medical Devices Regulation applies since 26 May 2021.

List of Current European Union Medical Device Regulations and... Download Scientific Diagram
REGULATION (EU) 2017/746 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL. of 5 April 2017. on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU (Text with EEA relevance) THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION,

(PDF) Regulation of medical devices outside the European Union
The following medical devices Directives were repealed and replaced by Regulation (EU) 2017/746 and Regulation (EU) 2017/745 respectively. New Regulations The EU revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years.
Preparing For The Future The New European Union Medical Devices Regulation pepgra
The Medical Device Coordination Group (MDCG) of experts have published guidance on a range of topics including the following: Borderline and classification. Clinical investigations and evaluations. Covid-19. Custom-made devices. EUDAMED. European Medical Device Nomenclature (EMDN) Implant cards. In Vitro Diagnostic medical devices (IVDs)

Medical Devices EU regulations for MDR Part 1 Cosmotrace
(1) Council Directive 90/385/EEC (3) and Council Directive 93/42/EEC (4) constitute the Union regulatory framework for medical devices, other than in vitro diagnostic medical devices. However, a fundamental revision of those Directives is needed to establish a robust, transparent, predictable and sustainable regulatory framework for medical devices which ensures a high level of safety and.

How MDR affected legislation of medical devices in European Union Member States Blog Healthmed
The European Commission has launched a probe to examine how China favours its domestic companies in tenders for medical devices and weigh possible tit-for-tat measures. #EuropeNews

(PDF) A Review on European Union New Medical Device Regulations2017
The European Union (EU) Medical Device Regulation (MDR) (2017/745) replaces the EU Medical Devices Directive, and establishes a regulatory framework for medical devices that safeguards public health and safety while supporting the competitiveness of the market. The EU MDR came into force on May 26, 2021. The regulation places restrictions and.

The impact of new European Medical Device Regulations MedTech Innovation
About. This site is intended as a Wiki for the 2017 European Union Medical Device Regulation (EU MDR). The primarily goal of the site is to provide a practical guide to compliance. To simplify the regulation for anyone wishing to supply compliant medical devices to European citizens. For those already supplying devices in compliance with the.

(PDF) The New European Medical Device Regulation 2017/745 Main Changes and Challenges
Purpose This manuscript presents a parallel among important EU legal frameworks, such as the Medical Device Regulations and General Data Protection Regulation and economic-historical challenges faced by European citizens. This parallel offers a prospective for understanding the direction taken by policymakers for forthcoming regulations, such as the European Health Data Space and the AI-Act.

Addressing the challenge of the new European Union Medical Device Reg…
Download MDR. Download from the link below the MDR in the main European languages. If you prefer the HTML with TOC version just look into the HMTL column ans select the version for your native language. Here is the direct link to MDR English version HTML with TOC. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April.

European Medical Device Regulation Guide to simplify compliance 2021
FDA vs. EU Medical Device Regulation RAM Technologies
medical devices for human use and accessories for such devices in the Union. This Regulation also applies to clinical investigations concerning such medical devices and accessories conducted in the Union. 2. This Regulation shall also apply, as from the date of application of common specifications adopted pursuant to Article 9, to the groups of
- Disney Plus Ya Funciona En Holanda
- 2019 Lexus Nx 300 Release Date
- Las Llaves De La Independencia
- Fazer Vista Grossa
- Frankfurt Salchichas Y Cervezas Insurgentes
- Fincas De Caza En Guadalajara
- Audios Conferencia Sobre Alimentos En Catalan
- Area Of Square Feet Formula
- Atheros Wireless Network Adapter Windows 10
- Rebasar La Velocidad Para Adelantar